There is considerable overlap of the bands arising from α-helical and the unordered conformations. It is therefore generally difficult to assign the bands appearing in this region. Recording an IR spectrum in D2O decreases this overlap to some extent. Dissolution a protein in D2O results in the exchange of solvent exposed amide protons by deuterium. Hydrogens of the unordered amides are more easily exchanged as compared to those involved in the secondary structures. Despite this, it is not easy to unambiguously assign the bands arising in the 1657-1648 cm-1 region. Circular dichroism and IR spectroscopy therefore complement each other wherein α-helices are easily detected by CD and β-sheets by IR. Like CD, an IR spectrum of a protein can also be deconvoluted to determine the fractions of different secondary structural elements as shown in Figure 10.8.
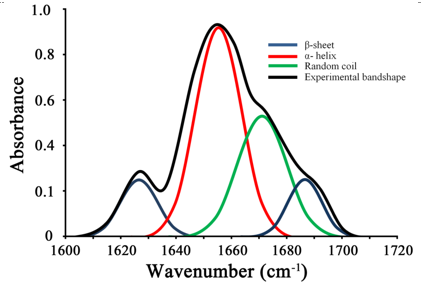
Figure 10.8. Deconvolution of Amide I band of a protein to identify the fractions of different structural elements
Box 10.1: Michelson interferometer A Michelson interferometer has a radiation source, a collimator, a beam-splitter, a movable mirror, a fixed mirror, a compensator, and a detector.
The radiation coming from the source is collimated and focused on the beam-splitter. 50% of the radiation gets transmitted while 50% gets reflected. The mirrors reflect back the radiation towards the beam splitter that again allows 50% transmission and 50% reflection. This allows the beams, B and C to interfere and give the beam D. As the beam, B travels through the beam-splitter twice while beam C does not travel through it even once, a compensation plate of same material (un-mirrored) and thickness as the beam-splitter is, is placed between the beam-splitter and the fixed mirror. This allows the beams, B and C to travel the equal distance. The motion of the movable mirror, M2 causes the two beams to travel different distances thereby generating interference. Let us see what happens when a monochromatic radiation is used in the Michelson interferometer. If the beams, B and C travel the equal distance, they are in phase and will interfere constructively. If however, the M2 moves, say towards the beam-splitter by a distance of |