Introduction
| Infrared (IR) region of the electromagnetic spectrum lies between visible and microwave regions and therefore spans the wavelengths from 0.78 – 250 μm. The energies associated with molecular vibrations are smaller than those associated with electronic transitions and fall in the IR region. IR spectroscopy, therefore, is used to probe the vibrations in molecules and is also known as vibrational spectroscopy. Infrared region is usually divided into three regions: near infrared, mid-infrared, and far infrared (Figure 10.1). IR spectroscopists use wavenumbers |
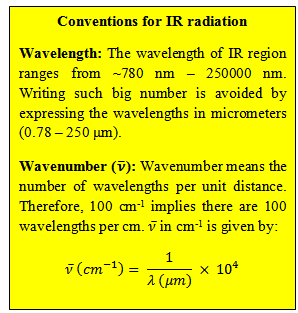 |
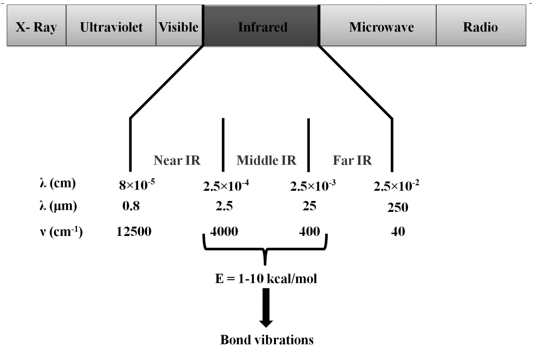
Figure 10.1 Infrared region of the electromagnetic spectrum |