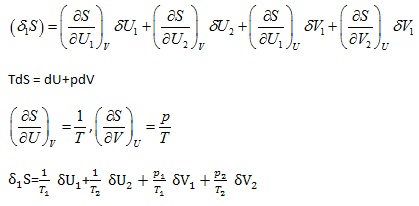A system is in a state of equilibrium if, for any finite variation of the system at constant T & p, G increases i.e. the stable equilibrium state corresponds to the minimum value of G.
A system is said to be in a state of neutral equilibrium when the thermodynamic criterion of equilibrium (G, F, S, U or H) remains at constant value for all possible variations of finite magnitude. If perturbed, the system does not revert to the original state.
For a system at constant T and P, the criterion of neutral equilibrium is
|
(2.111) |
Similarly
|
(2.112) |
A system is in a state of unstable equilibrium when the thermodynamic criterion is neither an extremum nor a constant value for all possible variations in the system. If the system is in unstable equilibrium, there will be a spontaneous change accompanied by
|
(2.113) |
A system is in a state of metastable equilibrium if it is stable to small but not to large disturbances. A mixture of oxygen and hydrogen is in a metastable equilibrium. A little spark may start a chemical reaction. Such a mixture is not in its most stable state, even through in the absence of a spark it appears to be stable.
Figure shows different types of equilibrium together with their mechanical analogies. S has been used as the criterion for equilibrium
Local equilibrium conditions
Let an arbitrary division of an isolated system be considered, such that
S = S1 + S2 , U = U1 + U2 |
(2.114) |
Then for equilibrium, it must satisfy the condition
|
(2.115) |
to first order in small displacements (otherwise ![]() could be made positive because of higher order terms). Now t the first order in a very small change
could be made positive because of higher order terms). Now t the first order in a very small change
|
(2.116)
|