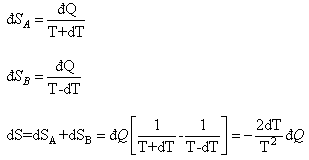A system is said to be in a state of stable equilibrium if, when the state
is perturbed, the system returns to its original state. A system is not in
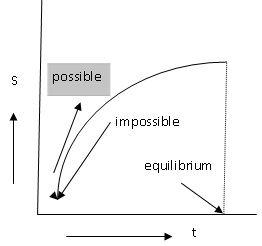
equilibrium if there is a spontaneous change in the state. If there is a spontaneous
change in the system, the entropy of the system increases and reaches a maximum
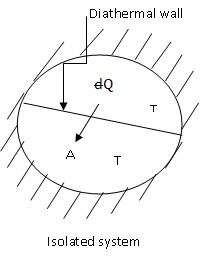
when the equilibrium condition is reached (fig. 2.11). Both A and B (fig.
2.12) are assumed to be at the same temperature T. Let there be some spontaneous
change; the temperature of A rises to T + dT, and that of B decreases to T
- dT. For simplicity, let the heat capacities of the bodies be the same, so
that difference of temperature dT is same for the both the systems A and B.
If 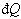 is the heat interaction involved, then the
entropy change
is the heat interaction involved, then the
entropy change
Fig. 2.11 |
Fig. 2.12 |
(2.107)
(2.108) |
so there is a decrease in entropy for the isolated system with A and B together. It is thus clear that the variation in temperature dT cannot take place. The system, therefore, exists in a stable equilibrium condition. Perturbation of the state produces an absurd situation and the system must revert to the original stable state. It may be observed:
if for all the possible variations in state of the isolated, there is a negative change in entropy, then the state is in stable equilibrium.
|
(2.109) |
Similarly
|
(2.110) |