3.13.2.5. Case Study of Biomimetic Polyene Cyclization
In contrasts the older method of synthesis which uses sequential annulations in a stepwise fashion, Biomimetic Polyene Cyclization is a method of synthesis utilizing a single reaction to form several rings with a cation-π mechanism.
Looking at the biosynthesis pathway of Squalene, Progesterone and other higher Sterol, it will be clear that the key steps of cyclization of linear polyenes involve the following steps (Figure 3.48). The general process of cyclase cyclization of linear polyenes:
- Generation of a carbocation
- Control of the conformation of the substrate
- Stabilization of intermediate
- Quenching of the final carbocation
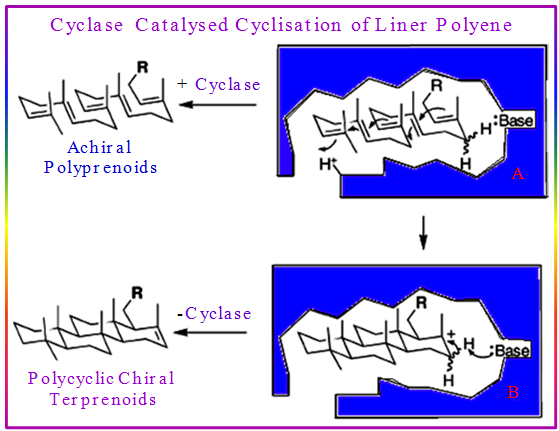
Figure 3.48: The general process of cyclase cyclization of linear polyenes.
The following facts are also further clear from the general process of cyclase cyclization of linear polyenes as well as from the squalene biosynthesis:
- Potential control of stereochemistry. One step produces four rings, 8 stereogenic centers and 1 out of 128 possible isomers.
- Readiness with which highly substituted carbon-carbon bonds may be formed.
- Only trans fused rings formed.
Squalene cyclization to lanosterol drew attention to various research groups. And Professor Adolf Windaus in his Nobel Lecture in 1928 rightly stated “The synthesis of such a substrate appears to the chemist particularly difficult, and up till now I have not dared to attempt it”.
However, it is the all time desire of chemists to recapitulate nature’s activity/productivity and then to mimic it. It is this everlasting desire that drive Professor Sir Robert Robinson to devise the route of biomimetic synthesis of troponone for the first time in the history of biomimetic synthesis who stated that “There has thus been a tendency to explain … that plants have…enormously powerful reagents that are able to cause substances… to undergo transformations which cannot be induced in the laboratory…….” And “To a certain extent…this must be true, but it is probable that this aspect has been exaggerated and that an equally important cause of the variety and complexity of syntheses in plants resides in the highly reactive nature of the substances which function as intermediate products.”
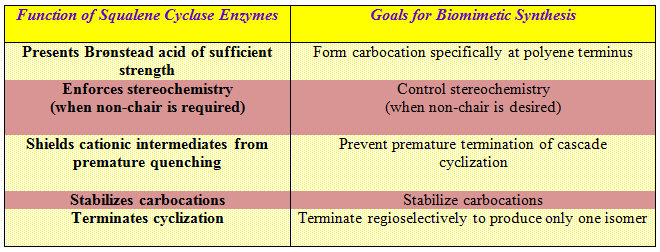
Corey et al. have correlated nicely between the function of squalene cyclase enzymes and the goals for biomimetic synthesis as has been stated below (Wendt, K.U.; Schulz, G.E.; Corey, E.J.; Liu, D.R. Angew. Chem. Int. Ed. 2000, 39, 2812-2833.)
The total synthesis of nature products in the earliest days were made successful by combining the available synthetic methods with a contemporary understanding of biosynthesis. Thus, in those days, biosynthetic considerations are largely inspiration for a synthesis route to a target. When a target’s biosynthetic route was unknown, total synthesis played an important role to inspire discrete hypotheses about a target's biosynthesis. However, there is now a clearer understanding of the mutual benefits. The ultimate outcome of mutually exclusive approach is the development of synthetic methodologies that merge upon the efficient techniques of nature. Biosynthetic hypotheses have also guided experiments to establish natural product structure.
In natural enzymatic system, enzymes are able to activate relatively unreactive substrates, such as simple olefins, to undergo multiple bond-forming reactions with high selectivity. In contrast to synthetic systems in which numerous pathways compete with carbocyclization, enzymes exert a miraculous level of control to produce just a few of these possibilities that ultimately lead to high selectivity for the overall transformation. The polyene carbocyclization provides an enzyme model for remarkable multifaceted selectivities in ring size, number of rings formed, stereochemistry of fused ring formation, and the degree of atom/group rearrangement.
Here, I am providing few examples of a case study in polyolefin cyclizations in which the biosynthesis−total synthesis interplay presents an incontrovertible synergism.
The milestone in Biomimetic Cyclization of Linear Polyenes follows:
- 1950s → Study of the stereochemistry of cationic cyclization of linear polyenes using simple model (Contribution from Stork and Eschenmoser)
- 1980s → Developing initiator, terminator and cation-stablizing group for cyclization, biomimetic pentacyclization (Contribution from Johnson)
- 1990s → Application of chiral LBAs in the enantioselective cyclization of linear polyenes (contribution from Yamanoto)