| |
| |
|
| |
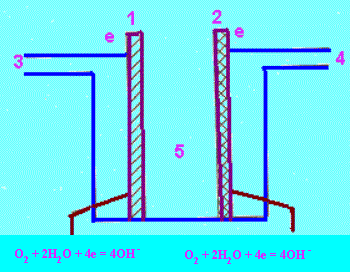
| Figure 23.4 : Schematic representation of a fuel cell (H2 - O2) using an alkaline electrolyte |
1. Porous Ni anode, 2] Porous Ni - NiO cathode, 3] H2 inlet, 4] O2 inlet, 5] Electrolyte, KOH (aq) |
| |
4 H2O + 4e- = 2H2+ 4OH- ; Eo = -O.83 V |
 2H 2 + O2 = 2H 2O ; Eo = + 1.23 V 2H 2 + O2 = 2H 2O ; Eo = + 1.23 V |
| |
In spite of higher efficiencies, fuel cells are rather expensive because of the difficult reaction conditions and the requirements of specific catalysts and electrodes. However they are used in space-crafts because of their light weight and also because the product of oxidation, water, can be used by astronauts. |
| |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
|