| The equilibrium constant. |
| For one mole of a gas A at any pressure P, the free energy is given by |
| |
| G A (p) = Go A + RT ln PA /Po |
(21.48) |
| where Po is the standard pressure of 1 bar and GoA is the standard free energy at 1 bar. When a reaction represented by the following equation takes place, (Ideal gas behaviour is assumed throughout this section). |
| |
| aA +bB = cC +dD
|
(21.49) |
where, a, b, c and d are the stoichiometric coefficients, and reaches equilibrium, the initial pressures of A, B, C and D change to the final equilibrium values (PA)eq , (PB)eq , (PC)eq , (PD)eq . At equilibrium, the free energy has reached the lowest value and there will be no further decrease and so 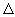 G = 0. The free energy change for eq.(21.49) can be written as G = 0. The free energy change for eq.(21.49) can be written as |
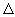 G = G products - Greactants G = G products - Greactants |
| |
| = c GC + d GD – aGA - bG B |
(21.50) |
| But cGC = cGoC + c RT ln (PC ) eq / P0 and similarly for A, B and D. Therefore, |
| |
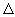 G = G = 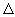 G0 + RT { ln{(Pc /P0) c (PD /P0) d / (PA /P0) a (PB /P0) b } G0 + RT { ln{(Pc /P0) c (PD /P0) d / (PA /P0) a (PB /P0) b } |
(21.51) |
Where  G 0 = c G 0C + d G0D – a G 0A – b G 0B . The ratio of pressures in eq (41.51) may be expressed as a factor Q. G 0 = c G 0C + d G0D – a G 0A – b G 0B . The ratio of pressures in eq (41.51) may be expressed as a factor Q. |