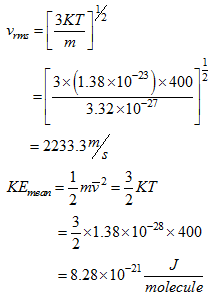
Much better agreement is achieved if f = 5. Then,
![]()
This implies that at room temperature, the diatomic molecules possess either
rotational or vibrational energy, but not both. At higher temperature, however,
both of these modes become active. At very low temperature, cv
approaches ![]() suggesting that only translational motion is present.
suggesting that only translational motion is present.
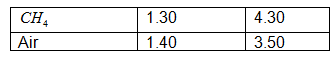
Minimum degree of freedom for a single molecule is f = 3, thus γ = 1
+ 2/3 = 1.67. For heavy molecules the degree of freedom is large thus 2/f
in the expression of γ approaches to 0 value. Hence γ must be
between 1 and 1.67.
Example 4.1:
Calculate the rms speed for hydrogen at 400 K. What is the mean translation kinetic energy of a molecule of hydrogen?
Solution:
Mass of an hydrogen molecule, m
![]()