1.10.6.2.1. Bioorthogonal Peptide Sequences
The tetracysteine-biarsenical system affords a powerful alternative to GFP tagging for protein visualization.
1.10.6.2.2. Ketones and Aldehydes
Ketones, and aldehydes are bioorthogonal chemical reporters that can tag not only proteins, but also glycans and other secondary metabolites.
1.10.6.2.3. Azides
The Staudinger Ligation
In contrast to aldehydes and ketones, azides are versatile chemical reporters for labeling all classes of biomolecules in any biological settings. The azides are good electrophiles subject to reaction with soft nucleophiles. This versatile functional group is absent in almost all naturally occurring species. Due to its wonderful bioorthogonality, recently the azide is being used as a chemical reporter in living systems. It is kinetically stable and contains large intrinsic energy. Thus, azides are prone to unique modes of reactivity. Therefore azide has been exploited for the development of bioorthogonal reactions, including the Staudinger ligation of azides with functionalized phosphines and the click reaction {[3+2] cycloaddition} with activated alkynes. These reactions can be used for the selective labeling of azide-functionalized biomolecules.
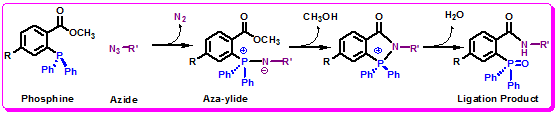
Figure 1.36: The Staudinger Ligation.
The Staudinger ligation has been used to modify glycans on living cells. Thus, glycoproteins are enriched with ligated components thereby, imparting new functionality to recombinant proteins.
Copper-catalyzed [3+2] azide-alkyne cycloaddition
Azides are also 1,3-dipolar in nature, thus, can undergo reactions with dipolarophiles such as activated alkynes. These π-systems are both extremely rare and inert in biological systems, thus, further increasing the bioorthogonality of the azide along the reaction with alkynes. More than four decades ago, the [3+2] cycloaddition between azides and terminal alkynes to provide stable triazole adducts was first described by Huisgen. The reaction is thermodynamically favorable. Without alkyne activation, however, the process requires stringent reaction conditions (high temperatures or pressures) which are incompatible with living systems. Therefore to make the process facile and thus, compatible with living systems, the alkyne must be activated. One possible way of activating alkynes is to attach an electron withdrawing functional groups like an ester; however, the resulting α,β-unsaturated carbonyl compounds can then act as Michael acceptors for a variety of biological nucleophiles. Therefore this is loosing bioorthogonality.
To make the azide-alkyne cycloaddition a bioorthogonal, one should activate alkyne via activating the terminal alkyne proton by using a catalyst like Cu (I). Thus, the Cu (I)-catalyzed azide-alkyne cycloaddition would be facile at biological temperature and also the rate of the reaction could be faster compared to uncatalysed Staudinger ligation.
Strain-Promoted Cycloaddition
Use of ring strain is an alternative means of activating alkynes for a catalyst-free [3+2] cycloaddition with azides. Constraining the alkyne within an eight-membered ring creates ~18 kcal/mol of strain. This strain energy is released in the transition state upon [3+2] cycloaddition with an azide. As a consequence, cyclooctynes react with azides at room temperature, without the need for a catalyst. Thus, this strain-promoted cycloaddition has been used to label biomolecules both in vitro and on cell surfaces without observable toxic effects. However, the reaction is limited by its slow rate.
1.10.7. How to Introduce Chemical Reporters in Cell’s Biomolecules?
In Proteins
To exploit the bioorthogonal chemistry of ketones, azides and alkynes (those functional groups are not present in any natural amino acids) for protein labeling we need using a cell’s translational machinery in either a residue-specific or a site-specific manner. Some of the techniques will be found in Module 2.
In Glycoconjugates
Azides can be incorporated into glycoconjugates using glycan biosynthetic pathways. Thus, as is shown in figure 34, azido analog of GlcNAc (GlcNAz) can be incorporated into cytosolic and nuclear glycoproteins.
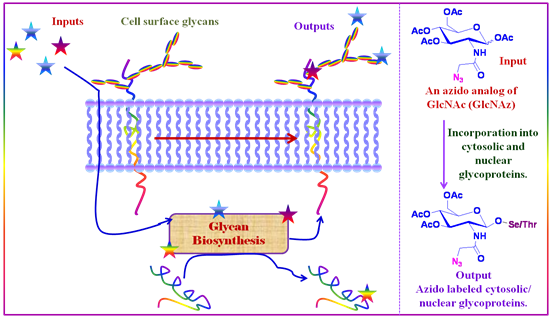
Figure 1.37: Strategy to incorporate Azides into glycoconjugates.