Temperature programmed oxidation TPO
Temperature programmed oxidation is used for investigation of the redox behavior of catalysts part icularly when applied in cyclic TPR-TPO experiments. The equipment and experimental procedure are same as TPR study. The only difference is that the sample should be in reduced form so that it is oxidized in the carrier gas containing oxygen. The carrier gas generally used is O2 /Ar mixture gas. Usually it is carried out after TPR and together gives a TPR-TPO cycle.
Temperature programmed desorption (TPD)
Temperature programmed desorption technique measures the desorbed molecules from the sample surface. In TPD experiments, catalysts are pretreated in situ in oxidative atmosphere at 100-2000C to remove any adsorbed species on the surface. Then, the sample is equilibrated with an adsorbing gas or vapor saturated with the probe molecule under well-defined conditions. After the excess gas is flushed out of the reactor, the sample is heated in a flowing inert gas stream. The concentration of the desorbing gas in the effluent gas is continuously monitored by a thermal conductivity detector. The total area under the curve gives the total amount of desorbed probe molecules. The same instrument used for TPR can be used for TPD study. For TPD, a mass spectrometer detector can also be used to detect the evolution of species from the surface back into gas phase. TPD spectra are affected by inert gas flow rate, particle size, catalysts pore size and catalysts bed depth.
Application
TPD studies gives:
- Type and amount of different forms of adsorbed species which correspond to the presence of various peaks
- Relative bond strength between the adsorbate and surface which corresponds to the various peaks at different temperature. Higher the desorption temperature stronger the bonds and site strength
TPD is extensively used for
- Study of acidic and basic sites on the surface
- Study of gas adsorbed on surface
- Study of organic compounds adsorbed on surface
For study of acidic and basic sites mainly two types of probe adsorbate molecules are used for TPD studies :
- NH3 desorption for determining acidic sites
- CO2 desorption for determining basic sites
Since NH3 is basic in nature it adsorbs on the acidic sites on the surface. The acidic sites are quantified in terms of total ammonia molecules adsorbing on the surface in μmol/gm of catalysts. However ammonia adsorption can not distinguish between Bronsted or Lewis acid sites and only determines the total acidic sites. CO2 is an acidic gas and can absorb on basic sites on catalysts surface. This technique also gives the total basic sites quantified by total CO2 molecules adsorbed on the surface in μmol/g of catalysts.
Adsorption study of any gases such as CO, H2, O2, CH4 on catalysts as required can easily be done using TPD. Total area under the curve gives capacity of adsorption and multiple peak positions gives the bond strength.
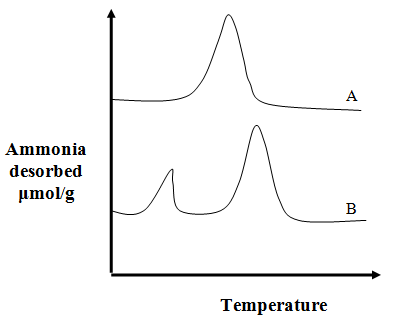
Fig. 5. Typical TPD spectra observed
Fig. 5 shows a typical NH3-TPD spectrum observed for different samples. Curve A only has one peak suggesting existence of one type of acidic sites on the sample to which ammonia is attached, while curve B has two peaks and suggests two types of acidic sites. The lower temperature peak suggests weaker bond strength and hence weaker acidic sites, while the peak at the higher temperature means stronger bonds and hence stronger acidic sites. The area under the curve gives the amount of weaker and stronger sites. For curve B, concentration of the stronger sites is more. Similar kind of conclusions can be drawn from CO2-TPD profiles corresponding to basic sites.