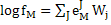 |
(4) |
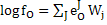 |
(5) |
Where J denotes all alloying elements. If steel contains C, and Mn.
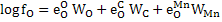 |
(6) |
Where e is interaction parameter
All oxide products are definite compounds except compounds formed by  deoxidation. Here product is either solid deoxidation. Here product is either solid 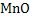 or liquid or liquid 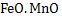 . .
In complex deoxidation where a mixture of 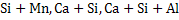 is used, the following advantages are reported as compared with simple one: is used, the following advantages are reported as compared with simple one:
- The dissolved oxygen is lower.
- Due to formation of liquid deoxidation product agglomeration of the product into large size can be obtained easily and can be floated easily.
According to equation 2
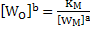 |
(7) |
Equation 7 indicates that weight percent oxygen in steel depends on value of 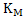 for small concentration of deoxidizers. At for small concentration of deoxidizers. At 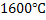 the value of Km is the value of Km is 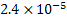 for the reaction for the reaction
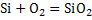 and for the reaction and for the reaction
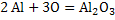
The value of 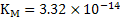 . Similarly for the reaction . Similarly for the reaction 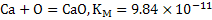 . The value of . The value of 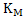 indicates the deoxidizing ability of an element. For the above reaction, calcium is the most efficient deoxidizer and indicates the deoxidizing ability of an element. For the above reaction, calcium is the most efficient deoxidizer and 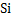 is not so efficient as compared to calcium. Aluminum is also a strong deoxidizing element when compared with silicon. is not so efficient as compared to calcium. Aluminum is also a strong deoxidizing element when compared with silicon.
Though calcium and aluminum are very efficient deoxidizers, but they oxidize very fast and moreover, their density is much lower than steel. Also 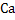 has a boiling point has a boiling point 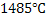 which means calcium is gaseous phase at the steelmaking temperature. Suitable injection methods or addition methods are to be developed. which means calcium is gaseous phase at the steelmaking temperature. Suitable injection methods or addition methods are to be developed.
|