Deoxidation of steel
Deoxidation can be carried out either by single element such as 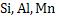 etc or by mixture of elements such as etc or by mixture of elements such as 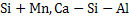 etc. Deoxidation by single element is known as simple deoxidation, whereas deoxidation by a mixture of elements is known as complex deoxidation. In both simple and complex deoxidation, oxide is formed; hence it is also termed precipitation deoxidation. Deoxidation is also carried out by carbon under vacuum; which is called vacuum deoxidation. Elements are added in the form of Ferro-alloys etc. Deoxidation by single element is known as simple deoxidation, whereas deoxidation by a mixture of elements is known as complex deoxidation. In both simple and complex deoxidation, oxide is formed; hence it is also termed precipitation deoxidation. Deoxidation is also carried out by carbon under vacuum; which is called vacuum deoxidation. Elements are added in the form of Ferro-alloys 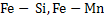 or or 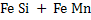 etc. etc.
Simple deoxidation can be represented by
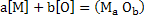 |
(1) |
If deoxidation product is pure then activity of 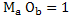 and if elements are in dilute solution and if elements are in dilute solution
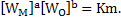 |
(2) |
Where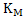 is deoxidation constant and equals to is deoxidation constant and equals to 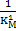 where where 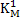 is equilibrium constant. is equilibrium constant.
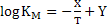 |
(3) |
Where X and Y are constants and T is temperature. Increase in T increases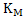 . Using equations 2 and 3 one can calculate the variation of . Using equations 2 and 3 one can calculate the variation of 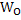 with with 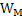 when when 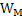 is in small quantity. For larger is in small quantity. For larger 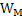 , interaction parameters need to be considered. , interaction parameters need to be considered.
|