Dissolution and homogenization of de oxidizer. Mechanism of dissolution depends on melting point. Ferro alloys melt at around
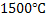
. Aluminium is expected to melt faster due to its much lower point. Intensity of agitation will govern the homogenization of deoxidizer in steel melt for faster kinetics of reaction between oxygen and deoxidizer.
Nucleation of solid product becomes easier if interface is present. Deoxidation by
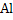
produces solid
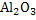
and as such
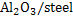
interface is useful for nucleation.
Growth of the deoxidation product: It depends on the state of the product. A liquid product can coalesce easily as compared with the solid product. Deoxidation with single elements like
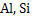
etc. produce solid deoxidation product at the steelmaking temperature. Deoxidation with ferro silicon+ ferro manganese produces liquid deoxidation product. Boron, titanium zirconium are also quite effective deoxidizers. Manganese and silicon are used in the ratio
7:1 to 4:1 in order to obtain a thin liquid slag.
Removal of deoxidation product: Removal of deoxidation product is equally important. It is achieved by floatation and absorption into a slag. Following steps are important for removal of de oxidation products from steel: