Home
Lecture
Quiz
Design Example |
Coagulation in Water Treatment
Destabilization using Al(III) and Fe(III) Salts
Jar Test
Jar Testing Apparatus
Jar Test Procedure
Coagulation in Water Treatment
-
Salts of Al(III) and Fe(III) are commonly used as coagulants in water and wastewater treatment.
-
When a salt of Al(III) and Fe(III) is added to water, it dissociates to yield trivalent ions, which hydrate to form aquometal complexes Al(H2O)63+ and Fe(H2O)63+. These complexes then pass through a series of hydrolytic reactions in which H2O molecules in the hydration shell are replaced by OH- ions to form a variety of soluble species such as Al(OH)2+ and Al(OH)2+. These products are quite effective as coagulants as they adsorb very strongly onto the surface of most negative colloids.
Destabilization using Al(III) and Fe(III) Salts
-
Al(III) and Fe(III) accomplish destabilization by two mechanisms:
(1) Adsorption and charge neutralization.
(2) Enmeshment in a sweep floc.
-
Interrelations between pH, coagulant dosage, and colloid concentration determine mechanism responsible for coagulation.
-
Charge on hydrolysis products and precipitation of metal hydroxides are both controlled by pH. The hydrolysis products possess a positive charge at pH values below iso-electric point of the metal hydroxide. Negatively charged species which predominate above iso-electric point, are ineffective for the destabilization of negatively charged colloids.
-
Precipitation of amorphous metal hydroxide is necessary for sweep-floc coagulation.
-
The solubility of Al(OH)3(s) and Fe(OH)3(s) is minimal at a particular pH and increases as the pH increases or decreases from that value. Thus, pH must be controlled to establish optimum conditions for coagulation.
-
Alum and Ferric Chloride reacts with natural alkalinity in water as follows:
Al2(SO4)3.14H2O + 6 HCO3-  2 Al(OH)3(s) + 6CO2 +14 H2O + 3 SO42- 2 Al(OH)3(s) + 6CO2 +14 H2O + 3 SO42-
FeCl3 + 3 HCO3-  Fe(OH)3(S) +3 CO2 + 3 Cl- Fe(OH)3(S) +3 CO2 + 3 Cl-
Jar Test
The jar test is a common laboratory procedure used to determine the optimum operating conditions for water or wastewater treatment. This method allows adjustments in pH, variations in coagulant or polymer dose, alternating mixing speeds, or testing of different coagulant or polymer types, on a small scale in order to predict the functioning of a large scale treatment operation.
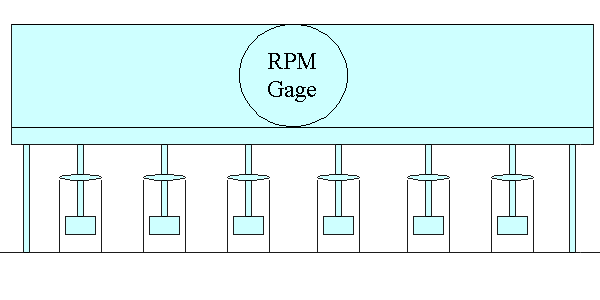
Jar Testing Apparatus
The jar testing apparatus consists of six paddles which stir the contents of six 1 liter containers. One container acts as a control while the operating conditions can be varied among the remaining five containers. A rpm gage at the top-center of the device allows for the uniform control of the mixing speed in all of the containers.
|
Jar Test Procedure
-
The jar test procedures involves the following steps:
Fill the jar testing apparatus containers with sample water. One container will be used as a control while the other 5 containers can be adjusted depending on what conditions are being tested. For example, the pH of the jars can be adjusted or variations of coagulant dosages can be added to determine optimum operating conditions.
-
Add the coagulant to each container and stir at approximately 100 rpm for 1 minute. The rapid mix stage helps to disperse the coagulant throughout each container.
-
Turn off the mixers and allow the containers to settle for 30 to 45 minutes. Then measure the final turbidity in each container.
-
Reduce the stirring speed to 25 to 35 rpm and continue mixing for 15 to 20 minutes. This slower mixing speed helps promote floc formation by enhancing particle collisions which lead to larger flocs.
-
Residual turbidity vs. coagulant dose is then plotted and optimal conditions are determined. The values that are obtained through the experiment are correlated and adjusted in order to account for the actual treatment system.
|
|

