5.13 Epoxides
Three-membered cyclic ethers are called as epoxides.
5.13.1 Synthesis
Alkenes can be converted to epoxides using peroxyacids (also called peracids) (Scheme 16). Use of strong acids should be avoided as it opens up the epoxides to give glycol.
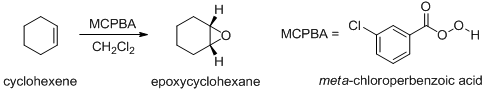
Scheme 16
The peroxy acid transfers an oxygen atom to the alkene in one one-step as shown below. The stereochemistry of alkene is maintained in the product (Scheme 17).
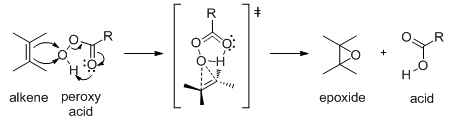
Scheme 17
Epoxides can also be obtained by the treatment of a halohydrin with a base (Scheme 18). Treatment of the trans -chlorohydrin with aqueous sodium hydroxide gives the epoxide through the internal SN2 reaction. This reaction can be used to synthesize cyclic ethers with larger rings.
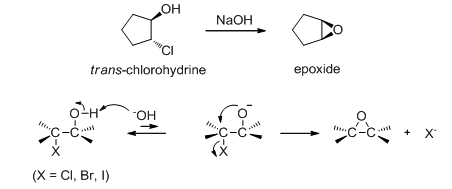
Scheme 18
Allylic alcohols can be converted into chiral epoxy alcohols with good enantioselectivity (Scheme 19). For example, the Sharpless epoxidation using tert -butyl hydroperoxide, titanium (IV) isopropoxide and (+)-diethyl tartrate catalyzes the epoxidation of geraniol with 95% ee.
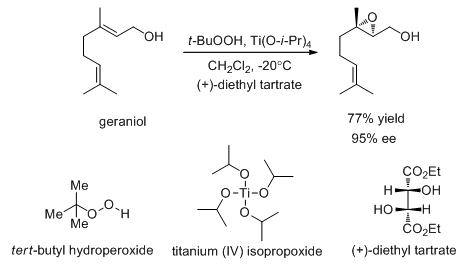
Scheme 19
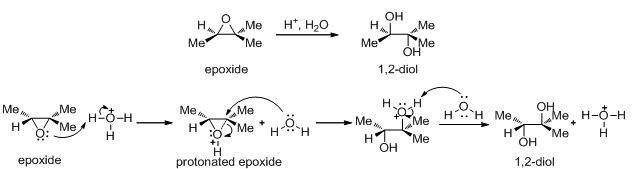
5.13.2 Reactions
The highly strained three-membered ring of epoxides makes them much more reactive toward nucleophilic substitution (Scheme 20). Acid catalysis assists epoxide ring opening as shown below. In the acid-catalyzed ring opening of an unsymmetrical epoxide the nucleophile attacks primarily at the more substituted carbon atom and this is SN1 like reaction. In the following example protonated epoxide is unsymmetrical which has a considerable positive charge on the more highly substituted carbon atom. The nucleophile, therefore, attacks this carbon atom even though it is more highly substituted.
Scheme 20