5.11.9 Curtius Rearrangement
An acid chloride reacts with azide ion to give an acyl azide, which undergoes Curtius rearrangement on heating to give an amine (Scheme 10).
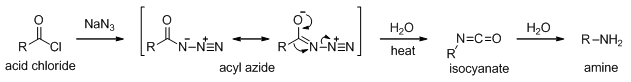
Scheme 10
5.12 Ethers
5.12.1 Nomenclature
- The common name of ether is given by citing the two groups attached to the ether oxygen in alphabetical order, followed by the word ether.
- In this IUPAC style, ethers are named as alkoxyalkanes, alkoxyalkenes, and alkoxyarenes.
5.12.2 Synthesis
The most reliable ether synthesis is the Williamson ether synthesis , in which an alkoxide ion attacks on a less hindered primary alkyl halide or tosylate, in SN2 fashion.
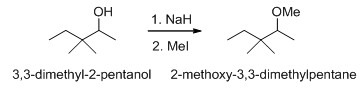
Scheme 11
Phenols can be reacted with alkyl halides to give alkyl aryl ethers in the presence of base such as K2CO3 under milder reaction conditions (Scheme 12).
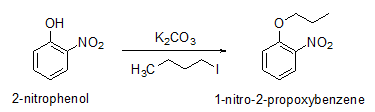
Scheme 12
Ethers can also be synthesized by alkoxymercuration–demercuration method (Scheme 13). The reaction of an alkene with an alcohol in the presence of a mercury salt leads to an alkoxymercury intermediate, which on reaction with sodium borohydride yields the ether.
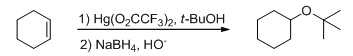
Scheme 13
A tert -butyl ether can be used to “protect” the hydroxyl group of an alcohol, which can then be easily removed by treating the ether with dilute aqueous acid. A hydroxyl group can also be protected by converting it to a silyl ether group. Usually tert -butyldimethylsilyl chloride is used to convert an alcohol to the corresponding silyl ether, although triethylsilyl chloride, triisopropylsilyl chloride, tert -butyldiphenylsilyl chloride and others can also be used (Scheme 14).
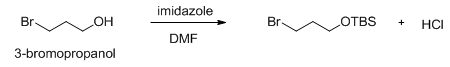
Scheme 14
5.12.3 Reactions
Diethyl ether reacts with hot concentrated hydrobromic acid to give two molecular equivalents of bromoethane (Scheme 15). An oxonium cation is formed in the first step which is then attacked by a bromide ion to produce an ethanol and ethyl bromide. Excess HBr reacts with the ethanol to produce the second molar equivalent of ethyl bromide.
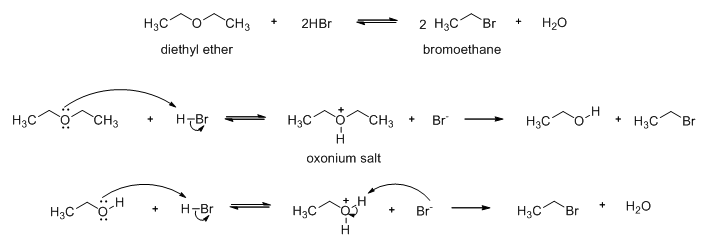
Scheme 15