1.3 Ionic, Covalent and Polar Bonds
According to Lewis's theory, an atom is most stable if its outer shell is either filled or contains eight electrons. So it will give up, accept, or share electrons in order to achieve a filled outer shell or an outer shell that contains eight electrons. This theory is called as octet rule . Lithium (Li) has a single electron in its 2s atomic orbital. The lithium atom ends up with a filled outer shell, a stable configuration, if it loses the 2s electron.
Energy released on removing an electron from an atom is called ionization energy. Lithium has relatively low ionization energy because loss of electron leads to stable configuration and become a positively charged. The elements in the first column of the periodic table, alkali metals, are all electropositive because they lose their outermost electron readily. Electrons in inner shells, called core electrons, do not participate in chemical bonding. Electrons in the outermost shell are called valence electrons, and the outermost shell is called the valence shell. Carbon, for example, has two core electrons and four valence electrons.
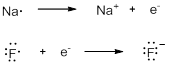
The chemical behavior of an element depends on its electronic configuration. Elements in the same column of the periodic table have the same number of valence electrons, and have similar chemical properties. When we draw the electrons around an atom, only valence electrons are shown as a dot. Sodium readily loses its valence electron to have stable electronic configuration and becomes positive ion. Fluorine gains one electron to achieve stable electronic configuration and becomes negative ion. Energy is released when an atom gains an electron.
1.3.1 The Ionic Bond
A chemical compound is called an ionic compound in which the components atoms exist as ions. Crystalline KCl results when potassium metal and chlorine gas are mixed. One electron is transferred from potassium atom to chlorine.
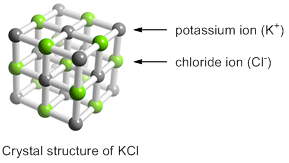
The positively charged potassium ions and negatively charged chloride ions are held together by the electrostatic attractions. An electrostatic attraction that holds ions together is called an ionic bond. Thus, the crystal structure of KCl is maintained by ionic bonds between potassium ions and chloride ions.