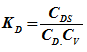Therefore, the controlling step can be either of the following:
(1) Surface reaction
(2) Adsorption
(3) Desorption
Now total concentration of active sites on surface, C0, will be the summation concentrations of all sites on which either reactants or products are adsorbed and the concentration of vacant sites.
![]()
Where CV is the concentration of vacant sites.
Case 1 : Rate is surface reaction controlling
The surface reaction is the slowest step and is the rate controlling. According to the mechanism, surface reaction occurs between adsorbed A and adsorbed B producing adsorbed C & adsorbed D.
![]()
The rate of surface reaction is given as
| .ks= rate constant for forward surface reaction | |
| |
............................................................................................................
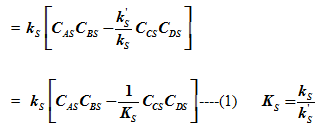 |
..................--- |
Now, since all the other steps are considered to be in equilibrium, therefore concentration of adsorbed species can be obtained as follows.
For adsorption steps and desorption steps :
From step (1)![]() from step (4)------
from step (4)------ 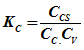
From step (2)![]() From step (5)-------
From step (5)-------