Accurate and precise determination of analytes
It is hardly necessary to explain how critical an accurate determination of an analyte is. If a breath alcohol detector is not accurate, a drunk driver may be let off risking the life of others while a sober one may be detained. Unless the concentration of analyte is determined accurately and precisely, it is difficult to make meaningful conclusions. So, what exactly do the accuracy and precision mean? Accuracy is the measure of how closely the measured values match the true values. Precision tells about the reproducibility of the measurement i.e. how closely the measured values are if repeated measurements are made on the sample (Figure 1.1).
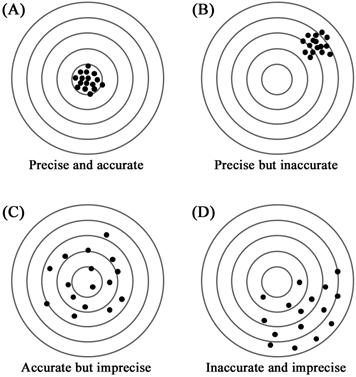
Figure 1.1 Schematic representations of accuracy and precision. Consider the centre of the concentric circles as the true value; the measured values are represented as the black dots. The measured values shown in panel A are close to the true value (accurate) as well as to each other (precise). The measured values in panel B are close to each other (precise) but far from the true value (inaccurate). The individual values in panel C are far away from the true value but randomly distributed about the true value; the average value lies close to the true value (accurate but imprecise). Panel D represents inaccurate and imprecise measurements.
It is easy to imagine the consequences of using an inaccurate equipment; it would give inaccurate results. Imprecise equipments, even if accurate, are problematic as a large number of measurements are required to arrive close to the true value which may take considerable amount of time. An analytical tool therefore has to be both accurate and precise to be used reliably and for faster analysis.