Exercise iii
Antimony can be produced according to the following reaction:
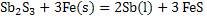
Calculate the following for a process in which 800 g of 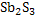 is mixed with 300g of Fe to form 250g of Sb is mixed with 300g of Fe to form 250g of Sb
a) The limiting reactant, b) percent excess reactant, c) the degree of conversion of Fe to  , d) percent conversion, e) the yield of Sb , d) percent conversion, e) the yield of Sb
Solution:
Limiting reactant is the one which controls the completion of reaction.
The stoichiometry of reaction suggests
800g of 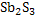 would require would require 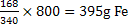 for the completion of reaction. But only 300g of for the completion of reaction. But only 300g of 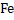 is mixed hence is mixed hence
a) Iron is limiting reactant
b) 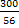 moles of Fe can reduce 1.79 moles of moles of Fe can reduce 1.79 moles of 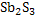
The available moles of 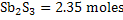 . .
Excess reactant 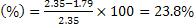
c) Degree of conversion of Fe to 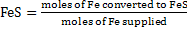
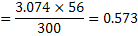
Or 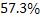 Answer © Answer ©
d) Yield of 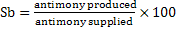
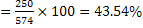
|