Metal extraction requirement:
The chemical combination of metal with sulphur or oxygen in a mineral is accompanied with high heat of formation
|
Heat of formation
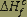 (k cal /kg mole) |
Fe2O3 |
198 x103 |
Al2O3 |
380 x103 |
Cu2s |
19 x103 |
ZnS |
44 x103 |
PbS |
22 x103 |
Fe3O4 |
26 x103 |
Ti O2 |
218 x103 |
MgO |
142 x103 |
|
Thus large amount of energy would be required for example to produce Fe or Al from their respective minerals. Two basic methods of metal extraction are:
Pyrometallurgical extraction
This method depends on whether oxide ore or sulphide ore is employed for metal extraction. In the extraction first step is to upgrade the metal grade by employing mineral beneficiation technologies. The concentrate is then mixed with a suitable reducing agent and the mixture is followed by smelting to separate gangue minerals form metal. Here large amount of energy is required to facilitate separation of metal from gangue.
Energy source and its consumption are important. For the sulphide ore the following route is employed.
Sulphide is first converted to oxide and then following by smelting. Zinc and lead are produced by this route.
In another route sulphides are used to obtain by matte by matte smelting. It is used to produce copper.
Hydrometallurgical extraction
Hydrometallurgical extraction is mainly suited to lean ores.In the hydrometallurgical extraction metal is brought in to solution by leaching, which is then followed either by hydrogen reduction or electrolytic reduction to obtain metal.
This route is used to produce Al and Mg.
|