Characterization of natural reserves of metals
The natural reserve of a metal is called “ore”. Ore is an aggregate of minerals. A mineral in an inorganic compound in which elements are mixed in stoichiometric proportion, for example Al2O3 is a mineral in which 2 moles of aluminum are combined with 1.5 moles of oxygen gas. An ore of any metal contains valuable mineral and gangue minerals. Valuable mineral is the mineral which is used to produce metal.
In the ore, metal grade is important.
Metal grade of an ore = 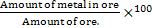
It must be noted very clearly that ore does not contain metal but metal in the ore is in the form of a mineral. Metal grade is used to characterize an ore reserve. For example metal grade of iron in pure
Fe2O3 is 70%. If iron ore contains 80% Fe2O3 , then iron grade of ore in 56%. This means that 44% of the ore is waste both in terms of solid and oxygen of the valuable mineral. In the following table metal grade of certain ores, valuable minerals etc. are given:
We note the following form the above table:
Metal |
Ore |
Valuable mineral |
Metal grade (%) |
Al |
Bauxite |
Al2O3 |
17.4%. |
Ti |
Ilmenite |
Ti O2 |
36% |
Cu |
Sulphide |
CuFeS2 |
2 to 3%. |
Fe |
Hematite |
Fe2O3 |
56% 64% |
Ni |
Sulphide ore |
Ni3S2 |
2.3% |
Pb |
Sulphide ore |
PbS |
5.5% |
|
i) Metal in the valuable mineral is chemically combined with either oxygen or sulphur.
ii) Metal grade in sulphide ore is very low as compared with oxide ores.
iii) Low grade of any ore means large production of wastage. Thus waste production is a part of metal production from natural reserves.
|