| |
Conditions for dephosphorization:
Dephosphorization requires oxidizing and basic slag:
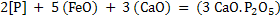 |
(9) |
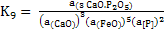 |
(10) |
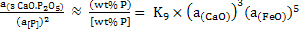 |
(11) |
-
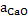 in slag should be high. This means slag should have free dissolved lime. in slag should be high. This means slag should have free dissolved lime.
High basicity of slag is required.
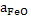 in slag should be high; slag should be oxidizing. However for efficient in slag should be high; slag should be oxidizing. However for efficient
dephosphorization the FeO content of slag should be in between 15 to 16%.-
Low temperature favours high 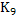 |
Conditions for simultaneous removal of C and P
Removal of C and P both require oxidizing conditions but P removal is possible only when a basic and limy slag is formed. Consider the following reactions occurring simultaneously
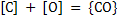 |
(12) |
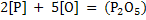 |
(13) |
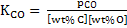 |
(14) |
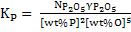 |
(15) |
It is assumed in eq. 14 and 15 that henrian activity is equal to (wt %). Both reactions
12 and 13 require oxygen but reaction 13 requires a slag which is basic in nature
in addition to oxygen. Thus, if carbon and phosphorus are to be removed simulataneously,
an important requirement is the availability of slag which acts as a sink for (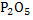 ). ).
Thermodynamically slag is required in which activity coefficient of 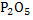 is very low. is very low.
The question is how low activity of 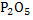 should be?. This value can be determined by should be?. This value can be determined by
equations 14 and 15
Replacing [wt% O] in equation 14 and 15, and after rearrangement,
|
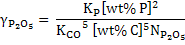
|
(16) |
|