|
The equation 9 can be used to determine wt% O in steel at any temperature T, when 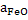 in slag is known. in slag is known.
When pure FeO is in contact with Fe;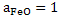 . We can determine . We can determine 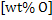 at saturation for different temperatures: at saturation for different temperatures:
T (K)
|
|
1873 |
0.233 |
1923 |
0.285 |
|
We note that increase in temperature increases oxygen dissolved in molten iron.
The above values of dissolved oxygen correspond when pure FeO is in contact with Fe pure. In steelmaking FeO is present along with other oxides like calcium oxide, magnesium oxide, silica, manganese oxide etc, hence activity of FeO is influenced by other solute oxides. Thus 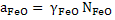
where 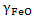 is activity coefficient and is activity coefficient and 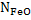 mole fraction of FeO in slag,. mole fraction of FeO in slag,.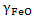 depends on slag composition. In CaO- depends on slag composition. In CaO-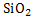 -FeO system, as -FeO system, as 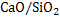 ratio increases ratio increases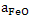 increases; physically it means that CaO replaces FeO from FeO. increases; physically it means that CaO replaces FeO from FeO.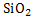 . The following expression is used to express . The following expression is used to express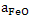 : :
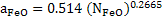 |
(10) |
Consider a slag with 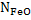 = 0.5 ; = 0.5 ; 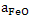 according to equation 10 is 0.31; [wt% O] in steel would be 0.072 as calculated by equation 9. according to equation 10 is 0.31; [wt% O] in steel would be 0.072 as calculated by equation 9.
Few other equations are available; i.e.
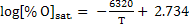 |
(11) |
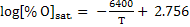 |
(12) |
The calculations are made on [wt% O] by equations 9, 11 and 12 at different temperatures using 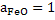 . .
T (K)
|

|

|

|
1873 |
0.233 |
0.229 |
0.220 |
1923 |
0.285 |
0.280 |
0.268 |
|
There is a slight difference in the values of dissolved oxygen content in steel. But all equations suggest that increase in temperature increases dissolved oxygen in iron which is in contact with pure FeO. This calculation indicates that control of temperature is important to limit the dissolution of oxygen in molten iron.
|