|
Conservation of Energy
The principle of conservation of energy for a control mass system is described by the first law of thermodynamics
Heat Q added to a control mass system- the work done W by the control mass system = change in its internal energy E
The internal energy depends only upon the initial and final states of the system. It can be written in the form of the equation as
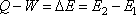 |
(13.1a) |
Equation (13.1a) can be expressed
on the time rate basis as
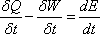 |
(13.1b) |
Where δQ and δW are the amount of heat added and work done respectively during a time interval of δt.
To develop the analytical statement for the conservation of
energy of a control volume, the Eq. (10.10) is used with N
= E (the internal energy) and η = e (the internal energy
per unit mass) along with the Eq. (13.1b). This gives
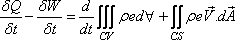 |
(13.2) |
The Eq. (13.2) is known as the general energy equation for a control volume.
The first term on the right hand side of the equation is the time rate of increase in the internal energy within a control volume and the second term is the net rate of energy efflux from the control volume.
Different forms of energy associated with moving fluid elements comprising a control volume are -
1. Potential energy
The concept of potential energy in a fluid is essentially the same as that of a solid mass. The potential energy of a fluid element arises from its existence in a conservative body force field. This field may be a magnetic, electrical, etc. In the absence of any of such external force field, the earth’s gravitational effect is the only cause of potential energy. If a fluid mass m is stored in a reservoir and its C.G. is at a vertical distance z from an arbitrary horizontal datum plane, then the potential energy is mgz and the potential energy per unit mass is gz. The arbitrary datum does not play
a vital role since the difference in potential energy, instead of its absolute value, is encountered in different practical purposes.
2. Kinetic Energy
If a quantity of a fluid of mass m flows with a velocity V, being the same throughout its mass, then the total kinetic energy is mV2/2 and the kinetic energy per unit mass is V2/2. For a stream of real fluid, the velocities at different points will not be the same. If V is the local component of velocity along the direction of flow for a fluid flowing through an open channel or closed conduit of cross-sectional area A, the total kinetic energy at any section is evaluated by summing up the kinetic energy flowing through differential areas as
The average velocity at a cross-section in a flowing stream is defined on the basis of volumetric flow rate as,
The kinetic energy per unit mass of the fluid is usually expressed as
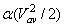 where
α is known as the kinetic energy correction factor. where
α is known as the kinetic energy correction factor.
Therefore, we can write
Hence,
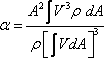 |
(13.3a) |
|