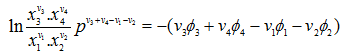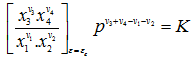Law of Mass Action
For a homogeneous phase chemical reaction at constant temperature and pressure, when the constituents are ideal gases, the chemical potential are given by the expressions of the type
|
(3.37) |
where the Φ's are functions of temperature only.
Substituting in the equation of reaction equilibrium (Eq. (1.38))
v1(Φ1 + ln p + ln x1)
+ v2(Φ2 + ln p + ln x2) |
(3.38) |
On rearranging
v3 ln x3 + v4 ln x4 - v1 ln x1 - v2 ln x2 + (v3 + v4 - v1 - v2)ln p |
(3.39) |
∴
|
(3.40) |
Denoting
ln K = -(v3Φ3 + v4Φ4 - v1Φ1 - v2Φ2) |
(3.41) |
where K, is known as the equilibrium constant, is a function of temperature only
|
(3.42) |
This equation is called the law of mass action. K has the dimension of pressure raised to the (v3+ v4- v1- v2)th power. Here the x's is the values of mole fractions at equilibrium when the degree of reaction is εε.
The law of mass action can also be written in this form
|
(3.43) |
where the p's are the partial pressures.