| Development of Electron Avalanche
|
| |
- Initially the electrons are originated in a gaseous dielectric gap space between two electrodes either by ionization of neutral molecules by photons from cosmic rays, or by ultraviolet illumination of cathode, or at a later stage by photons from the discharge itself when electric field is applied.
- The electrons thus generated accelerate towards the anode, gaining kinetic energy of movement from the applied electric field between the electrodes.
- The kinetic energy thus acquired by the electrons can be so high that on collision with neutral molecules it may ionize them (elastic collision) or render them to a higher excited or vibrational state (inelastic collision).
|
- When an electron gains more energy than required for ionization of the gas molecules (Table 6.1), then it is capable of ionizing, that is, ejecting an electron from the neutral molecule, and leaving behind a positive ion.
- The new electron thus ejected along with the primary one repeat the process of ionization.
- Since a molecule is much heavier compared to an electron, it can be considered relatively stationary, making no contribution to the ionization process.
- On the contrary, the electrons move very fast under the influence of applied electric field and continue to release further electrons from the gas molecules.
- An 'avalanche' of electrons finally reaches the anode as shown in Fig. 7.1
|
|
Fig 7.1 Development of an electron avalanche in uniform field |
- At the field intensities at which impact ionization occurs, the value of drift velocity of electrons in air is usually ~107 cm/s, while of positive ions it is about 150 times lower ~105 cm/s. Accordingly, the transit time required by electrons and ions to cross a gap differ about 150 times.
- The process of avalanche form of charge carrier multiplication was first described by Townsend(1901). Later he also gave its mathematical formulation.
- If only the process of electron multiplication by electron collision is considered in uniform field between two plates, Fig. 7.2, then neglecting other processes (recombination and diffusion), the number of electrons produced by collision at an element dx, at distance x from the cathode is,
|
| dnx= nx α dx (7.1) |
|
Fig. 7.2 Electrons in a uniform field |
where x is the distance form the cathode, α the Townsend's primary ionization coefficient, and nx the number of electrons at distance x from the cathode.
- In a uniform field where the field intensity E is constant, the ionization coefficient α can be considered constant. By integrating Equation 7.1 and applying the initial condition nx =n0 at x = 0, the following equation is derived for a uniform field,
|
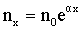 (7.2) (7.2) |
- For weakly nonuniform fields, where α is not constant, the above equation is written as,
|
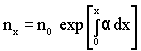 (7.3) (7.3) |
where n0 is the number of electrons emitted per second from the cathode, also known as the initial number of electrons.
|
- Therefore, in case of very small gap distances, the number of electrons striking the anode per second (at x = d) are,
|
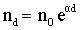 (7.4) (7.4) |
- This means that on an average each electron leaving the cathode produces (eα d - 1) new electrons and the same number of positive ions in traversing the distance d.
- The expressions 7.2 - 7.4 show distinctly the exponential or avalanche form of growth of the number of charge carriers by primary or α process.
- The Townsend's first ionization coefficient α is a function of the electric field intensity E, and at constant temperature it is dependent upon the gas pressure p. It can be proved that,
|
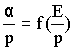 |
- The coefficient α can be calculated with the help of molecular parameters. However, α is usually obtained experimentally by measuring the multiplication of electrons in high electric fields. For air, the following equation is approximated [2.5].
|
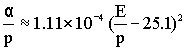 (7.5) (7.5) |
where E is in V/cm, p in Torr and α in cm-1.
- Equation 7.5 is plotted for α/p and Elp in Fig. 7.3 at constant temperature.
- Raether was able to take first photographs of the trace of an electron avalanche in 1939 [2.6]. He used the so called 'Wilson Cloud Chamber', which caused condensation of water vapour droplets on the charge carriers of an avalanche at appropriate gas pressure.
|
|
Fig 7.3 Apparent ionization coefficient α / p as function of E/p for air. |
- In his experiment, a short duration voltage pulse was applied on the electrode system shown in Fig 7.4 (a,c).
- When the voltage applied reached its desired peak value, just sufficient to develop an avalanche, it was maintained at this magnitude, but was not allowed to lead to a breakdown.
- Just at this stage, primary electrons were produced in the electrode system with the help of an external spark discharge source as shown in Fig. 7.4 (b).
- This gave rise to the development of an electron avalanche.
- In order to avoid a breakdown, the process was controlled by a steep reduction in the applied voltage to zero after the duration of a few tens of nanoseconds.
|
|
Fig 7.4 Experimental arrangement to produce an avalanche. |
- Since the drift velocity of electrons is about 150 times more than that of the ions, hence as soon as an avalanche is formed, the positive ions remain practically stationary where they are produced, i.e, at the tail of the avalanche.
- The head of the avalanche is consequently built-up by electrons.
- The form of the track is wedge shaped, apparently due to the thermal diffusion of the drifting electron swarm having acceleration in the direction of electric field.
- The head of the avalanche is rounded since the diffusion of electrons takes place in all directions.
- Fig 7.5 shows the distribution of charge carriers and actual photographs of avalanche [2.7, 2.8].
|
|
Fig 7.5 An electron avalanche in uniform field.
(a) Distribution of charge carrier and the shape,
(b) Actual photograph by Raether[ 2.7] |
- The experiments conducted by Wagner [2.8] in 1966 were on slightly longer gap distances in uniform field. He also photographed the electron avalanche in a cloud chamber.
- It was revealed that when the light was first detected in the chamber, the number of charge carriers in the avalanche, that is the space charge was insufficient to cause any distortion in the applied electric field E0.
- The center of the electron cloud moved at the electron drift velocity corresponding to the field E0.
- This light first detected is known as 'primary avalanche' shown in Fig. 7.5 (b).
- Through these experiments it was concluded that only after further development, when the total number of electrons reach ~107 to l08, the space charges of ions and electrons in the avalanche become strong enough to distort the applied field E0.
|