The conformational analysis of ethane is given below. Thus, between these two extremes, there are lots of other conformations and energy change is gradual is nature. However, this does not mean that the ethane spends equal time in all conformations. In fact it mostly stays at the bottom of the potential well (staggered conformation). Obviously, it comes to mind whether the hydrogen atoms are bulky enough to cause a change in the energy states of the eclipsed and staggered conformation. As a matter of fact, this occurs due to the fact that the electrons in the bonds repel each other and this repulsion is at a maximum in the eclipsed conformation. There may be some
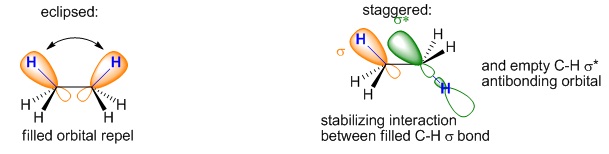
Figure 6
stabilizing interaction between the C–H σ-bonding orbital on one carbon and the C-H σ* anti-bonding orbital on the other carbon, which is greatest when the two orbitals are exactly parallel: this only happens in the staggered conformation (Figure 6).
As the hydrocarbon chain size increases, more complex effects seem to effect the energy considerations of the conformations (Figure 7). In the conformational study of propane, the conformational analysis can be done either along the C1-C2 bond or the C2-C3 bond-both being identical. In this case, the rotational barrier being 14 kJmol-1 is only slightly more than that of ethane. Thus the conformational analysis diagram is almost similar to ethane.
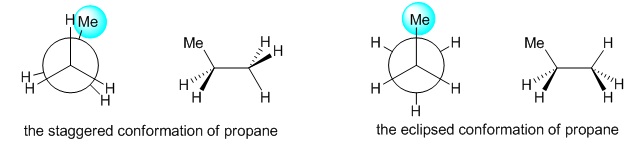
Figure 7
With butane, there are two methyl groups if we consider the C2-C3 bond as the pivotal bond for rotation. Here, the two methyl groups could eclipse each other in a conformation and since the steric hindrance due to this should be significant enough, so the potential energy will be highest for this conformation. The other eclipsed conformations will have a methyl group eclipsed by hydrogen which will be lower in energy than the former. Similarly, for the staggered conformations, there will two types of staggered conformations differing in energy. Hence the terms eclipsed and staggered are insufficient to describe the conformations.
A new system of naming the conformational isomers is thus devised (Figure 8). The term torsion angle is defined as the angle (having an absolute value between 0° and 180°) between bonds to two specified groups, one from the atom nearer (proximal) to the observer and the other from the further (distal) atom in a Newman projection. The torsion angle between groups A and D is then considered to be positive if the bond A-B is rotated in a clockwise direction through less than 180° in order that it may eclipse the bond C-D: a negative torsion angle requires rotation in the opposite sense. Stereochemical arrangements corresponding to torsion angles between 0° and ±90° are called syn (s), those corresponding to torsion angles between ±90° and 180° anti (a). Similarly, the arrangements corresponding to torsion angles between 30° and 150° or between -30° and -150° are called clinal (c) and those between 0° and 30° or 150° and 180° are called periplanar (p). The two types of terms can be combined so as to define four ranges of torsion angle; 0° to 30° synperiplanar (sp); 30° to 90° and -30° to -90° synclinal (sc); 90° to 150°, and -90° to -150° anticlinal (ac); ±150° to 180° antiperiplanar (ap). The synperiplanar conformation is also known as the syn- or cis-conformation; antiperiplanar as anti or trans and synclinal as gauche or skew.