7.1 Introduction
Michael Faraday isolated a pure compound from the oily mixture in the year of 1825. Elemental analysis evidenced hydrogen-to-carbon ratio of 1:1, corresponding to an empirical formula of CH. In 1834, Eilhard Mitscherlich synthesized the same compound by heating benzoic acid, isolated from gum benzoin, in the presence of lime. Like Faraday, Mitscherlich found that the empirical formula was CH. A vapor-density measurement showed the molecular weight of about 78, for a molecular formula of C6H6. He named it as benzin, since it was derived from gum benzoin and now it is called, benzene. Many compounds discovered in the nineteenth century seemed to be related to benzene. These compounds also had low hydrogen-to-carbon ratios as well as pleasant aromas. This group of compounds was called aromatic because of their pleasant odors. Other organic compounds without these properties were called aliphatic, meaning "fatlike." August Kekulé, the originator of the structural theory, suggested that the carbon atoms of benzene are in a ring. They are bonded to each other by alternating single and double bonds, and one hydrogen atom is attached to each carbon atom.
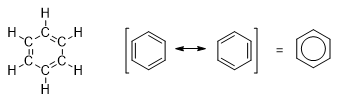
7.2 Structure
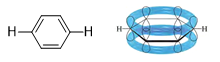
Benzene is a ring of six sp2 hybrid carbon atoms, each bonded to one hydrogen atom. It is a resonance hybrid of the two Kekule structures. The π-electrons are delocalized, with a bond order of 1.5 between adjacent carbon atoms. That is the carbon-carbon bond lengths in benzene are shorter than typical single-bond lengths, yet longer than typical double-bond lengths. All the carbon-carbon bonds are the same length, and all the bond angles are 120°. The unhybridized p-orbital of eachsp2 carbon atom is perpendicular to the plane of the ring and overlap to form a ring.
7.3 Properties
Benzene is a very stable than alkenes so benzenes do not undergo reaction that alkenes do. We know that an alkene decolorizes potassium permanganate by reacting to form a glycol. But when permanganate is added to benzene, no reaction occurs (Scheme 1).
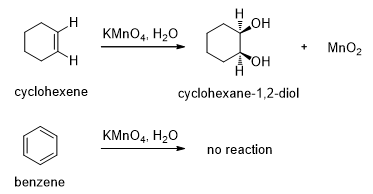
Scheme 1
In the same way, most alkenes decolorize solutions of bromine in carbon tetrachloride. The red bromine color disappears as bromine adds across the double bond. When bromine is added to benzene, no reaction occurs, and the red bromine color remains unchanged (Scheme 2).
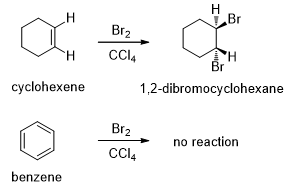
Scheme 2
By comparing heats of hydrogenation of benzene, cyclohexene, and cyclohexadiene, we can get an idea about the stability of benzene (Figure 1). On hydrogenation all these compounds give cyclohexane.
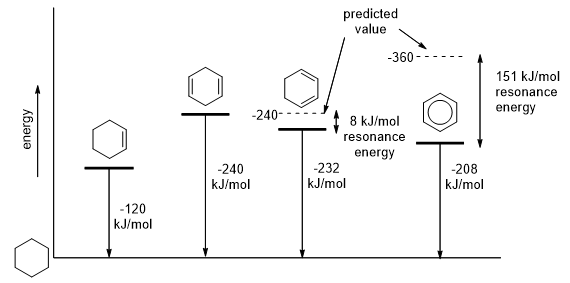
Figure 1
Hydrogenation of cyclohexene is exothermic by 120 kJ/mol. Hydrogenation of 1,4-cyclohexadiene is also exothermic by 240 kJ/mol which is about twice the value of the heat of hydrogenation of cyclohexene as predicted. So the resonance energy of the isolated double bonds in 1,4-cyclohexadiene is about zero.
Hydrogenation of 1,3-cyclohexadiene is exothermic by 232 kJ/ which is about 8 kJ/mol less than the predicted value of 240 kJ/mol. So the resonance energy of the conjugated double bonds in 1,3-cyclohexadiene is 8 kJ/mol. Hydrogenation of benzene requires higher pressures of hydrogen and active catalysts. This hydrogenation is exothermic by 208 kJ/mol, which is about 151 kJ/mol less than the predicted value of 360 kJ/mol.