When a quaternary ammonium hydroxide is heated, β -elimination takes place to give an alkene (Scheme 10). This elimination reaction of a quaternary ammonium hydroxide is called the Hofmann elimination.
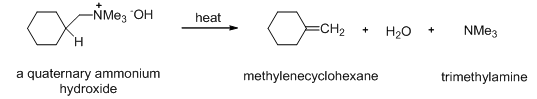
Scheme 10
The Hofmann elimination is an E2 elimination reaction where a proton and a tertiary amine are eliminated as shown in Scheme 11.
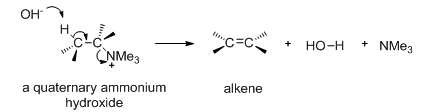
Scheme 11
The stereochemistry of Hofmann elimination can be explained by 2-butanamine. A base tends to abstracts a proton from β -carbon. In 2-butanamine there are two β-carbon, one of them has one methyl branch and the other one has no such branch. Base abstracts hydrogen from β-carbon which has no branch (Scheme 12).
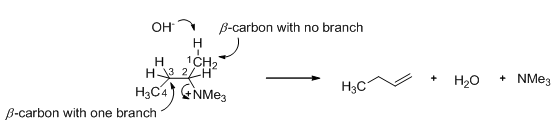
Scheme 12
E2 elimination requires anti -coplanar arrangement of the proton and the leaving group. Consider the possible conformations of the transition state for elimination at the C2-C3 bond that give a minor product (a more substituted alkene). Although conformers I and III are suitable for anti-eliminations, they contain repulsion between methyl and trimethylammonium group. Conformer II does not have anti -coplanar arrangement of the proton and the leaving group (Figure 8). The relatively higher energy of these conformers causes the corresponding reactions to be relatively slow.
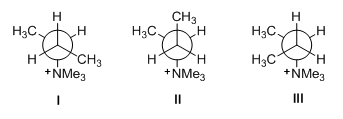
Figure 8
In contrast, elimination at the C1-C2 bond can occur with anti -stereochemistry because it has less steric repulsion and anti -coplanar arrangement of the hydrogen and the leaving (Figure 9). The crowded conformations of the transition state ( I , II and III ) are required for Zaitsev elimination, which are relatively high energy.
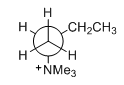
Figure 9
5.11.5 Oxidation
Tertiary amines can be oxidized to amine oxides by oxidizing agents such as H2O2 or a peroxyacid. This amine oxide can undergo the Cope elimination, much like the Hofmann elimination of a quaternary ammonium salt, because of the positive charge on nitrogen. The Cope elimination generally gives the same orientation as Hofmann elimination, resulting in the least-substituted alkene (Scheme 13).
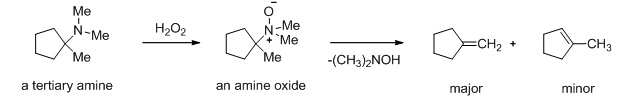
Scheme 13
An internal elimination takes place, where amine oxide has both the base and the leaving group, through a cyclic transition state with syn stereochemistry. In this case, either alkenes can be formed, but the major product would be the alkene which is less substituted (Scheme 14).
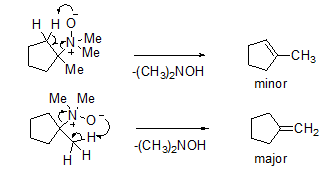
Scheme 14