1.8.3. Controlling the Reactivity of Myers-Saioto Cyclisation
Research into the Myers-Saito mechanism has focused on accessing the highly reactive enyne allene intermediate. In many systems its formation is sufficient to drive cyclization, as it releases approximately 15 kcal/mol upon conversion to the σ,![]() biradical. As a consequence of its great reactivity, several methods to mask and unveil the allene have been developed.
biradical. As a consequence of its great reactivity, several methods to mask and unveil the allene have been developed.
1.8.3.1 pH Dependent Myers-Saito Cylization
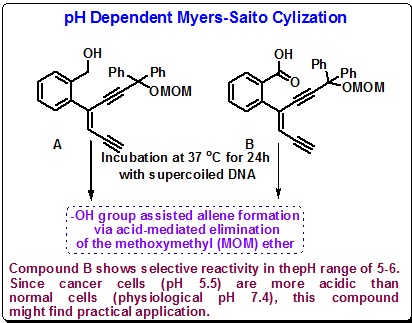
One promising result was observed upon incubating enediyne systems A and B at 37 oC for 24h with supercoiled DNA (Scheme 41). In this reaction, the hydroxyl group is believed to induce allene formation via acid-mediated elimination of the methoxymethyl (MOM) ether. No rationale for the differing reactivities between A and B was provided. Particularly pH range of 5-6. Since cancer cells (pH 5.5) are more acidic than normal cells (physiological pH 7.4), this compound may prove to be of practical importance.
 |
Scheme 41. pH dependent Myers-Saito cylization. |