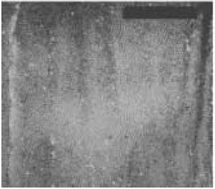 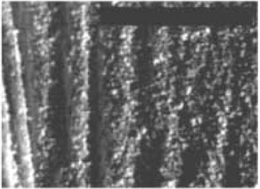
Figure 1. Optical image of a layer of hydrophobic particles spread on water. The layer is compressed which leads to the appearance of the wavy interface.
Many technological and medical applications involve particle-covered liquid interfaces, e.g. liquid drop coated with hydrophobic powder which turns the drop non-wetting with the result that the liquid marble can freely roll on a rigid surfaces or even float on water. Similarly, by encapsulating drugs within a monolayer of colloidal particles it may be possible to administer more medicines by inhalation and thus the efficacy of drug delivery. Figure 1 shows a monolayer of hydrophobic particles sprinkled densely onto an air-water interface. Such a monolayer can be formed by particles of wide range of sizes: 2.5 µ m – 6mm. The figure shows that the layer buckles when subjected to sufficient static compressive loading, suggesting that it can support an anisotropic stress. Such a stress can only be supported by a material with a non-zero shear modulus, which is the signature of the solid. Once the compressive stress is removed, the monolayer returns rapidly to the undeformed state, ironing out the wrinkles formed by the buckling. The solid like behaviour is observed only in the presence of the liquid, because, if the liquid is evaporated, it turns to powder without any cohesion. Clearly capillary forces are responsible for the formation of the solid monolayer via the aggregation of particles at the interface and act as the restoring mechanism when the monolayer is deformed.
|