1.2 One electron atoms
In an atom that contains a single electron, the potential energy depends upon the distance between the electron and the nucleus as given by the Coulomb equation. The Hamiltonian thus takes the following form:
(1.7) |
In atomic units this takes the form
(1.8) |
For the hydrogen atom, the nuclear charge, Z, equals +1. r is the distance of the electron from the nucleus. The helium cation, He+, is also a one-electron atom but has a nuclear charge of +2. As atoms have spherical symmetry it is more convenient to transform the Schrodinger equation to polar coordinates r, θ and Φ, where r is the distance from the nucleus (located at the origin), θ is the angle to the z axis and Φ is the angle from the x axis in the xy plane (See figure 1.1 below). The solutions can be written as the product of a radial function R(r), which depends only on r, and an angular function Y(θ,Φ) called a spherical harmonic, which that depends on θ and Φ.
(1.9) |
The wavefunctions are commonly referred to as orbitals and are characterised by three quantum numbers n, m and l. The quantum numbers can adopt values as follows:
n: principal quantum number: 0,1, 2,...
l: azimuthal quantum number: 0,1, ... (n - 1)
m: magnetic quantum number: —l, — (l — 1),... 0... (l — 1), l.
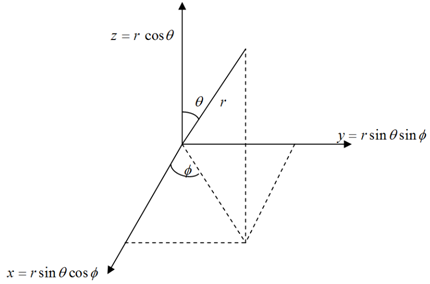
Figure 1.1: Relationship between spherical polar and Cartesian coordinates
(Adapted from Leach,2001)