Figure 38.4: Transformation by CaCl2 method.
Lab Experiment 38.2 : Transformation the vector into the yeast.
Background Information: The surface chemistry of yeast cells are different from the E.coli and as a result different methods to deliver DNA.
1. Lithium Acetate/ssDNA/PEG Method: In this method, yeast cells are incubated with a transformation mixture of lithium acetate, PEG 3500, single stranded carrier DNA and foreign plasmid at 420C for 40mins. The purpose of adding carrier DNA is to block the non-specific sites on cell wall and made plasmid available for uptake. Post-transformation, cells are pelleted to remove transformation mixture and re-suspended in 1ml water. It is plated on a solid media with an appropriate selection pressure such as antibiotics.
2. Spheroplast Transformation Method: In this method, yeast cell wall is removed partially to produce spheroplast. Spheroplasts are very fragile for osmotic shock but are competent to takes up free DNA at high rate. In addition, polyethyl glycol (PEG) is used to facilitate deposition of plasmid and carrier DNA on cell wall for easier uptake. The mechanism of DNA uptake in yeast is not very clear. A schematic of spheroplast method is given in Figure 38.5. (1) In the spheroplast method, yeast cells are incubated with zymolyase to partially remove cell wall to produce spheroplast. (2) They are collected by centrifugation and incubated with carrier DNA and plasmid DNA for 10mins at room temperature. (3) It is now treated with PEG and calcium for 10mins with gentle shaking. (4) Transformed spheroplast are plated on selective solid media and incubated on 300C for 4 days.
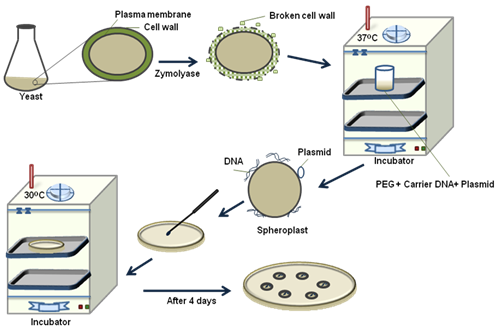
Figure 38.5: Steps in yeast transformation by sphereplast method.