Heat of formation
The formation of chemical compound from its elements is associated with either absorption or liberation of heat. Thus the formation of 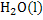 form the elements at 298K form the elements at 298K 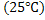 can be written can be written
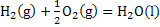
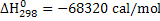 at 1 atm pressure at 1 atm pressure
Heat of reaction
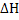 for any process depends only on the initial and final states and not on the path. If a process is divided into several steps, and for any process depends only on the initial and final states and not on the path. If a process is divided into several steps, and 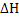 is determined for each step, the algebraic sum of the is determined for each step, the algebraic sum of the  values of all steps will be equal to values of all steps will be equal to 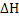 for the original process. Consider an example to calculate heat of reaction of the following reaction: for the original process. Consider an example to calculate heat of reaction of the following reaction:
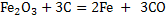 (15) (15)
This reaction comprises of the following reactions:
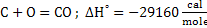
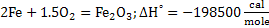
Heat of formation for reaction 15 at 298K is
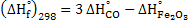
It must be noted that heat of formation of element is zero by using the value of 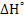 we get we get
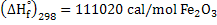 (endothermic), that means reaction 1 is accompanied by absorption of heat. (endothermic), that means reaction 1 is accompanied by absorption of heat.
Consider the reaction
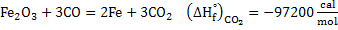
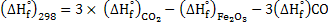
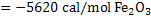 . This is exothermic reaction. . This is exothermic reaction.
The heat of reaction calculated above are at 298K 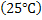 . Heat of reaction depends on temperature and can be determined . Heat of reaction depends on temperature and can be determined
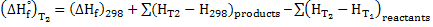 (16) (16)
Conclusion
This lecture discusses the basics of thermo chemistry as required to calculate the different types of heat associated with a chemical reaction. Heat of formation, latent heat of fusion, evaporation and transformation are discussed and illustrated with a suitable example. Effect of temperature on heat of reaction is also given. For details the readers may go through the references.
References:
Schuhmann : Metallurgical Engineering Principles
Alan and Geige r : energy balance in metallurgical processes
|