| |
Consider the following gasification reaction
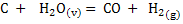
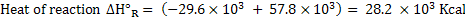
In the gasification of coal or coke with a mixture of air and steam, it is important to know how much amount of steam can be fed without supplying any heat from outside. Heat balance can yield the amount of steam which can be fed.
Consider gasification of 1 Kg mole of C with a mixture air and steam under adiabatic conditions:
Let X Kg mole of C reacts with air. Assuming that all steam decomposes to H2 and carbon forms CO.
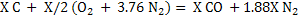 (2) (2)
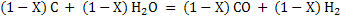 (3) (3)
Heat produced by eq. 2 = Heat consumed by eq. 3
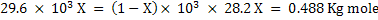
Therefore final equations for gasification becomes
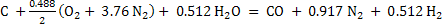 (4) (4)
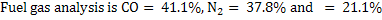
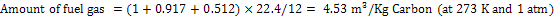
Amount of steam is 0.768 Kg/kg of carbon
Alternatively a mixture of oxygen and steam can also be used for gasification.Consider the gasification of a mole of carbon by a mixture of pure oxygen +steam
Assuming that all steam decomposes to 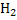 and carbon forms CO. and carbon forms CO.
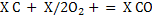 (5) (5)
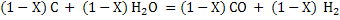 (6) (6)
Heat produced by eq. 5 = Heat consumed by eq. 6
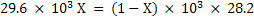
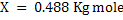
Therefore final equations for gasification becomes
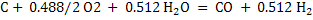 (7) (7)
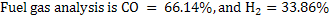
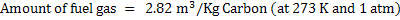
Amount of steam is 0.768 Kg/kg of carbon
|