Preamble
In lecture 31 a simplified material balance is considered for ironmaking blast furnace and the results of material balance formulation are presented in the form RIST diagram.. In that it is considered that charge consists of pure iron oxide and coke and the product is hot metal containing carbon only. We have not considered heat balance for that simple case.
This lecture considers heat balance in terms of heat input and output. Heat input is required to meet the thermal demand of the process of ironmaking. Heat demand of the blast furnace is met principally by the combustion of coke. In the calculations of coke rate, it is therefore necessary to consider the heat requirements. In the following we will be considering a simplified case in which pure oxide is a source of iron supply and coke is the source of carbon to illustrate the concept. This means that slag formation in this simplified case does not occur.
Enthalpy balance in blast furnace
Consider a case where input is pure 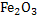 ( in reality iron ore is charged)and carbon (actually coke is used) at ( in reality iron ore is charged)and carbon (actually coke is used) at 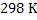 . Air is supplied at . Air is supplied at 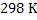 (in reality air is preheated). Hot metal is considered to be a molten mixture of iron and carbon and exits at (in reality air is preheated). Hot metal is considered to be a molten mixture of iron and carbon and exits at 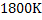 . At the moment we exclude slag formation. Top gas leaves at . At the moment we exclude slag formation. Top gas leaves at 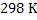 (this is an ideal case to explain the concept). (this is an ideal case to explain the concept).
Enthalpy balance
Enthalpy into furnace per mole of product 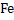 =Enthalpy out of furnace per mole of product =Enthalpy out of furnace per mole of product 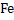 . .
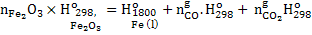 |
(1) |
,Where
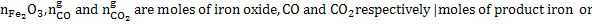
Or
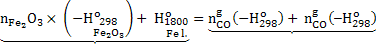 |
(2) |
Heat demand Heat supply
Negative sign in equation 2 indicates that heat is produced. In the equation 2 heat is required to raise the temperature of hot metal from 298K to 1800K. Heat of formation of 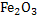 , CO and CO2 is given below: , CO and CO2 is given below:
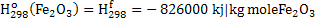
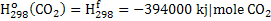
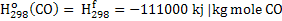
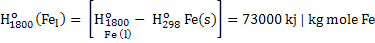 . .
Substituting the values in equation 2 we get
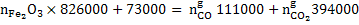 |
(3) |
|