Exercise –II Do Yourself
Hematite ore of 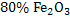 is reduced in blast furnace using coke of is reduced in blast furnace using coke of 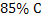 . .
The reduction equation is:
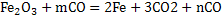
It is required to produce exit gas of composition 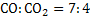 .The pig iron analyzes 94% and 4% C and ignore rest. .The pig iron analyzes 94% and 4% C and ignore rest.
Determine:
- Reduction equation, balanced with whole numbers
- Amount of coke/ton of pig iron
- Amount of air required/ton of pig iron to burn C of coke to produce CO
- % composition of gas resulting due to combustion and reduction
The readers should test themselves, how far have they understood. Purposely answers are not given.
Solution and answer can be found in video lecture number 31 on materials and heat balance in metallurgical processes.
|