| |
Preamble
Slag plays a very important role in steelmaking to the extent that it is said that “make a slag and slag makes steel”. Slag is a generic name and in steelmaking it is mostly a solution of oxides and sulphides in the molten state and the multi-crystalline phases in the solid state.
Slag is a separate phase because
- It is lighter than molten steel and
- It is immiscible in steel
Slag is formed during refining of hot metal in which Si oxidizes to 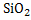 , Mn to MnO, Fe to FeO, and P to , Mn to MnO, Fe to FeO, and P to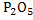 , and addition of oxides such as CaO, MgO, iron oxide, and others. The addition of oxides is done to obtain desired physico-chemical properties of slag like melting point, basicity, viscosity etc. All these oxides float on the surface of the molten steel. Synthetic slag is also used to absorb inclusions to produce clean steel for certain applications. , and addition of oxides such as CaO, MgO, iron oxide, and others. The addition of oxides is done to obtain desired physico-chemical properties of slag like melting point, basicity, viscosity etc. All these oxides float on the surface of the molten steel. Synthetic slag is also used to absorb inclusions to produce clean steel for certain applications.
|