1) Problem of desulphurization
a) Calculate 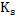 for a synthetic slag composed of for a synthetic slag composed of 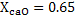 and and 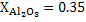 . The slag is contact with molten steel at temperature T. with dissolved aluminum . The slag is contact with molten steel at temperature T. with dissolved aluminum 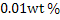 . In slag activity of alumina is 0.38. use . In slag activity of alumina is 0.38. use 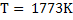 and and 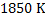 and interpret the results. and interpret the results.
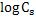 by equation 4 of lecture 22 = - 1.96. by equation 4 of lecture 22 = - 1.96.
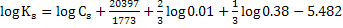
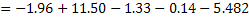
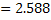
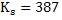 at at 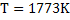
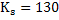 at at 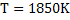
We note the partition coefficient is higher at 1773K as compared with that at 1850K. Ws can conclude that lower tempearure favours desulphurization.
b) Discuss the transitory and permanent contact mode of desulphurization based on the following calculations: Molten steel is desulphurised by injecting powder at the rate of 4 kg/ton. The partition coefficient of sulphur is 50. Now we select a slag whose partition coefficient is 400. We also increase the powder injection rate in one case to 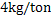 and in other case to and in other case to 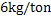 . What will be the effect of a) sulphur partition coefficient and b) powder injection rate on desulphurization modes . What will be the effect of a) sulphur partition coefficient and b) powder injection rate on desulphurization modes
Note a Similar problem is solved in lecture 23.
|