| |
Deoxidation practice
Deoxidation can be carried out during tapping, in ladles runners and even in moulds. Bath stirring is important. During tapping, bath is stirred due to potential energy but this subsides towards the end. When deoxidation is carried out in ladle, it is called ladle deoxidation in industrial practice. Depending on the extent of deoxidation, killed, semi killed and rimming steels are produced. For carbon content less than 0.15% and enough oxygen in steel, rimming steel can be produced.
Alloy steels are fully killed to obtain maximum recovery of alloying additions.
Illustration
Let us take an example of deoxidation of steel with ferromanganese. Manganese is a weak deoxidizer. We intend to reduce the dissolved oxygen in steel from 0.045 wt.% to 0.018 wt.%. How much manganese would be required?
Consider the reaction
(MnO) = [Mn] + [O]; 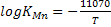 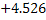
We can write 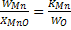 , we have assumed hMn= WMn and ho = Wo, where Wo is weight percent , we have assumed hMn= WMn and ho = Wo, where Wo is weight percent
Substituting the value of KMn = 0.0413 1t 1600 and Wo = 0.018 we get and Wo = 0.018 we get
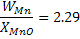
For the deoxidation with manganese, the following reaction must also be considered
(MnO) + [Fe] = [Mn] +(FeO); 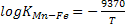 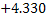
By writing equilibrium constant and using the value of 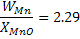 we can find XFeO we can find XFeO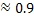 and WMn = 2.061 wt. percent. and WMn = 2.061 wt. percent.
Total manganese required would be equal to manganese required to remove (0.045% ⎯ 0.018%) oxygen + Manganese required to raise the content of mn from 0.1% to 2.061%
Calculation gives 20.53Kg Mn/ton steel. If the manganese content of ferromanganese is 60%, ferromanganese would be 34 kg/ton of steel
Reference:
A. Ghosh: Principles of secondary
processing and casting of steel.
K.W. Lange: Thermodynamics and kinetic
aspects of secondary steelmaking. International Materials Reviews, 1988, vol.
33 p 53
|