Gas radiation
In gas radiation, one has to distinguish between the gases which are transparent to radiation and which emit and absorb radiations. All diatomic gases like nitrogen and oxygen are transparent to radiation that means they neither emit nor absorb radiation at low temperatures. Whereas, tri-atomic gases like 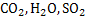 absorb and emit radiation to a considerable degree so that they are important in heat exchange absorb and emit radiation to a considerable degree so that they are important in heat exchange
Absorption and emission of gases differ from liquids and solids in the following ways.
i Gases emit and absorb radiation within a narrow band of wavelength and
ii Emission and absorption take place through the body of the gas. No. of modules of a gas is important for radiation. No. of molecules of gas at a given temperature is proportional to partial pressure of gas and size of the body 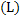 of the gas, where. of the gas, where.
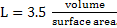 |
(15) |
Heat transfer between gas and enclosure can be given by
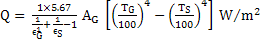 |
(16) |
|