| |
6.11.1 Cubic Spinel Ferrites
Cubic spinel ferrites have a formula AB2O4 which crystallize with a face centered cubic structure. (See Module 1). In these structures, two cations occupy tetrahedral and octahedral sites in an FCC lattice made by O atoms. One unit-cell consists of eight formula units of AB2O4 hence containing a total of 32 octahedral interstices with one fourth occupancy and 64 tetrahedral interstices with one eighth occupancy by the cations.
Depending on how these cations are distributed in the interstices, cubic spinel structures can be of two types:
Normal spinel and Inverse spinel
We won’t go through details of the structure as we have already done that in module 1. From the magnetism point of interest, the cations occupying tetrahedral sites have their spins oppositely oriented with respect to the cations on octahedral sites (up and down depends upon your frame of reference).
Compounds with normal spinel structure are ZnFe2O4, MgAl2O4, CoAl2O4 where A atoms occupy the tetrahedral sites while B atoms occupy the octahedral sites.
In compounds with inverse spinel strcture e.g. Fe3O4, NiFe2O4, half of B cations occupy tetrahedral sites and all A and the remaining 50% B cations occupy octahedral sites.
So, if we take the example of Fe3O4 which is actually Fe2+Fe3+2O4,
then the arrangement of spin is like what is shown below.
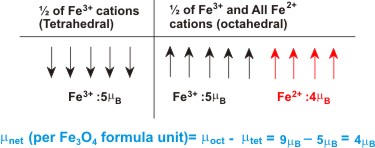 |
Hence the net magnetization of Fe3O4 is 4μB per formula unit which is quite a large magnetic moment. You can convert this into A.m-1 by simply calculating the net moment for the whole unit cell and dividing that by the cell volume.
Similarly you can work out magnetic moments of other ferrites such as NiFe2O4, CoFe2O4 etc. This approach is also valid for mixed spinel compounds.
Mixed spinels are quite a nice way of increasing the net magnetic moment. As you can see that since the moments of two sites are antiparallel, reducing the net magnetic moment of one site would actually increase the net moment. So mixing of NiFe2O4, an inverse spinel, and ZnFe2O4, a normal spinel, results in maximization of moment up to ~40 mol% ZnFe2O4.
In general, spinel ferrites show low magnetic anisotropy i.e. dependence of magnetization on crystallographic directions, and are magnetically soft i.e. show low coercive fields. Exceptions could be Co-containing ferrites which are not only strongly magnetically anisotropic but also show large coercive fields strengths. These materials also exhibit a ferromagnetic material like hysteresis loop when placed in a varying magnetic field.
|