The Barometer
Barometer is used to determine the local atmospheric pressure. Mercury is employed in the barometer because its density is sufficiently high for a relative short column to be obtained. and also because it has very small vapour pressure at normal temperature. High density scales down the pressure head(h) to repesent same magnitude of pressure in a tube of smaller height.
Fig 4.3 A Simple Barometer
Even if the air is completely absent, a perfect vacuum at the top of the tube is never possible. The space would be occupied by the mercury vapour and the pressure would equal to the vapour pressure of mercury at its existing temperature. This almost vacuum condition above the mercury in the barometer is known as Torricellian vacuum.
The pressure at A equal to that at B (Fig. 4.3) which is the atmospheric pressure
patm since A and B lie on the same horizontal plane. Therefore, we can write
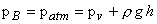 |
(4.1) |
The vapour pressure of mercury pv, can normally be neglected in comparison to patm.
At 200C,Pv is only 0.16 patm, where patm =1.0132 X105 Pa at sea level. Then we get from Eq. (4.1)
For accuracy, small corrections are necessary to allow for the variation of r with temperature, the thermal expansion of the scale (usually made of brass). and surface tension effects. If water was used instead of mercury, the corresponding height of the column would be about 10.4 m provided that a perfect vacuum could be achieved above the water. However, the vapour pressure of water at ordinary temperature is appreciable and so the actual height at, say, 15°C would be about 180 mm less than this value. Moreover. with a tube smaller in diameter than about 15 mm, surface tension effects become significant.
|