| |
Property
To describe a system and predict its behaviour requires a knowledge of its properties and how those properties are related. Properties are macroscopic characteristics of a system such as mass, volume, energy, pressure and temperature to which numerical values can be assigned at a given time without knowledge of the past history of the system. Many other properties are considered during the course of our study.
- The value of a property of a system is independent of the process or the path followed by the system in reaching a particular state.
- The change in the value of the property depends only on the initial and the final states.
The word state refers to the condition of a system as described by its properties.
Mathematically, if P is a property of the system, then the change in the property in going from the initial state 1 to the final state 2 is given by
If P = P (x, y) then,
where,
If 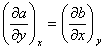 , then dP is said to be an exact differential, and P is a point function. A thermodynamic property is a point function and not a path function. Pressure, temperature, volume or molar volume are some of the quantities which satisfy these requirements. , then dP is said to be an exact differential, and P is a point function. A thermodynamic property is a point function and not a path function. Pressure, temperature, volume or molar volume are some of the quantities which satisfy these requirements.
|