NO Formation is a Function of Temperature and O2
From Eq. 2.11 the initial rate of NO formation when [NO]/[NO]e<< 1;
The concentration of atomic oxygen at equilibrium, using reaction ½ O2 ↔ O is given by;
Equilibrium constant K p(O) is
Using value of k1 from Table 2.2 and, the Eqs. 2.13 and 2.14 the initial rate of NO formation then reduces to
Temperature being in exponential term in Eq.2.15, it strongly influences NO formation rates. From Eq. 2.15 it follows that the NO formation is maximized under the conditions of high temperature and high oxygen concentrations. These conditions occur at fuel-air equivalence ratios 5-10% leaner than stoichiometric mixture.
Typical combustion duration is about 1 to 2 ms in SI engines operating close to stoichiometric conditions at 3000 -5000 rpm. NO formation at peak pressure and temperature conditions may reach close to equilibrium [NO] concentrations as illustrated below;
The characteristic time (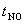 ) necessary to reach equilibrium concentration of NO may be approximated as, ) necessary to reach equilibrium concentration of NO may be approximated as,
The mole fraction [NO]e can be estimated from the reaction O2 + N2 ↔ 2NO, as
|