From Eq. (2.11) it is clear that higher the interaction energy between the two materials, the lower will be the interfacial tension.
From Eq.(2.1), energy of adhesion is,
This shows that adhesion of two surfaces in an inert gas or vacuum is always energetically (thermodynamically) favorable.
Therefore, knowledge of intermolecular interactions can help us to calculate 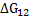
.
If in between there is another condensed phase 3 then,
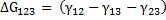
|
(2.13) |
Note that unlike in the case of two phases, here 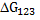 can be either positive or negative. can be either positive or negative.
|