We start with the particles placed far apart and bring them closer till we reach the secondary minimum. When particles come together in a secondary minimum, it is called flocculation. Then we further decrease the distance to reach the primary minimum and this is termed as coagulation.
Consider another example (Fig. 1.7). Here the forces are always attractive, even up to the primary minimum. So this dispersion would not be thermodynamically stable until it reaches the primary minimum . But still it could be kinetically stable because viscous resistance could resist the coagulation and it could take a very long time to reach the primary minimum. If 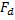 is the drag force on a particle and is the drag force on a particle and 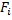 is the interaction force, then for such a (kinetically stable) system, is the interaction force, then for such a (kinetically stable) system,
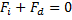
|
(1.2) |
Even though forces between two atoms or molecules are very weak and persist to short distances, summing up of these forces on the volume of colloidal particles can generate enough muscle to manifest even macroscopic behavior.
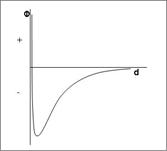
Figure 1.7. Another example of an energy function
|