Ostwald Ripening
This phenomenon is closely related to Kelvin equation.
As from Kelvin equation, it is clear that a smaller drop will have a higher saturation pressure in comparison to bigger drop hence the smaller one has a tendency to dissolve and bigger one to grow on expense of smaller one. So, one can conclude that smaller crystals have higher solubility.
Similar incident one notices in catalyst substrate surface where small drops of catalyst merge or agglomerate to form bigger drops and this leads to drop in surface area of catalyst.
This tendency also explains the self-stabilizing nature of a disturbed surface if it is left for a long time.
Bubbles (“) in liquid (‘)
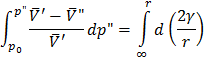
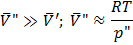
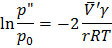
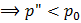
This is also not stable equilibrium.
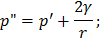
So to form a bubble we reduce 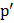 , which causes cavitations. , which causes cavitations.
Table 4.6
Radius of nuclei (nm) |
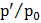
|
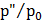
|
1000 |
1.001 |
0.999 |
100 |
1.011 |
0.98 |
10 |
1.12 |
0.9 |
1 |
3 |
0.3 |
So it is clear that for a radius >1000 nm, equilibrium conditions do not change drastically.
|