What is Colloid?
Colloid is a system containing entities having at least one length scale in between 1 nm and 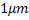 (Fig. 1.1). The word colloid itself originates from a Greek word meaning ‘glue-like’. (Fig. 1.1). The word colloid itself originates from a Greek word meaning ‘glue-like’.
Figure 1.1 Range for the colloidal phenomena
Behavior of systems falling in this range depends on the thickness (dimensions) of the system. When the dimensions become small enough the behavior of the system begins to deviate substantially from what we observe in case of larger dimensions. This is the reason why the study of colloids needs to be done separately and their behavior cannot be expected to be similar to those of macroscopic objects.
To understand this, we consider a spherical particle of radius 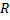 (Fig 1.2). As the particle size is reduced, the surface area to volume ratio increases as (Fig 1.2). As the particle size is reduced, the surface area to volume ratio increases as 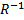 . For particles small enough, like in colloids, this ratio becomes significantly large and a higher percentage of molecules lie at the surface. Thus, surface properties become very important. . For particles small enough, like in colloids, this ratio becomes significantly large and a higher percentage of molecules lie at the surface. Thus, surface properties become very important.
Here as the size of the particle decreases, number of molecules on the surface increases in comparison to the bulk.
Figure 1.2 Effect of particle size on surface area
|